Research - American Journal of Preventive Medicine and Public Health (2023)
Chlorhexidine as a Therapeutic Agent for COVID-19 Infection
Y Hanna Huang1, Michael K Liu2 and Jong T Huang32College of Letters & Science, University of California, Berkeley, USA
3Department of Infectious Diseases, Good Samaritan Hospital, Los Angeles, United States
Received: 22-Sep-2023, Manuscript No. AJPMPH-23-114541; Editor assigned: 25-Sep-2023, Pre QC No. AJPMPH-23-114541 (PQ); Reviewed: 10-Oct-2023, QC No. AJPMPH-23-114541; Revised: 16-Oct-2023, Manuscript No. AJPMPH-23-114541 (R); Published: 23-Oct-2023
Abstract
Background: We previously documented that 0.12% chlorhexidine oral rinse and oropharyngeal spray could eliminate oropharyngeal SARS-CoV-2 which might prevent COVID-19 infection and also prevent SARS-CoV-2 spread from a patient to other people. We currently further investigated such application of chlorhexidine might also decrease mortality of COVID-19 infection.
Methods: We reviewed electronic medical records on patients we previously did study for chlorhexidine to eliminate oropharyngeal SARS-CoV-2 and determine whether chlorhexidine treatment influencing the mortality rate. We also repeated the same study in different period of COVID-19 infection to further confirm the efficiency of chlorhexidine in the management of COVID-19 infection. 0.12% of chlorhexidine was given oral rinse plus oropharyngeal spray for the initial 4 days of hospitalization. We also evaluated the possibility of persistence of SARS-CoV-2 in the oropharynx influencing the prognosis of COVID-19 infection.
Findings: The 1st study period for the year of 2020 through early 2021, 215 patients were enrolled in the study. The 2nd study was for the period of late 2021 through early 2022, 45 patients entered the study. They were divided into no chlorhexidine treated and chlorhexidine treated group. The study showed chlorhexidine treatment significantly cut the COVID-19 mortality to about half. For the study of SARS-CoV-2 persistence effect on the mortality, we demonstrated that disappearance of SARS-CoV-2 from oropharynx in 4 days after hospitalization significantly cut mortality to 61.9% in the group without chlorhexidine treatment which is statistically significant but only 28.8% in the chlorhexidine treated group which is not statistically significant.
Interpretation: Our previous and current chlorhexidine study provides that 0.12% chlorhexidine oral rinse and oropharyngeal spray may play an important role in preventing the COVID-19 infection and also reduce mortality of COVID-19 infection. The persistence of oropharyngeal SARS-CoV-2 is an ominous sign for the prognosis of COVID-19 infection.
Keywords
Antiviral agents; Coronavirus; Disinfectants; Dissemination; Epidemiology; SAR-CoV-2
Introduction
We previously published a paper demonstrating that chlorhexidine acts as an antiviral agent in eradicating SARS-CoV-2 from the oropharynx [1]. The study was conducted with the purpose of using chlorhexidine to prevent COVID-19 infection through contagious exposure and to prevent the further spread of SARS-CoV-2 from infected patients to others. Recently, we undertook a further investigation to review the potential effect of chlorhexidine in the management of COVID-19 infection. The study focused on the effect of chlorhexidine treatment in reducing the mortality rate of COVID-19 patients.
Materials and Methods
Study-1 method
We reviewed medical record for the patients in our previously published study [1]. Those patients were seen from 2020 through the early period of 2021.
The retrospective chart review for those patients was labeled as study 1: There were 215 patient charts available for review through Electronic Health Records (EHRs). The EHRs recorded that 117 patients were treated with 0.12% chlorhexidine via oral rinse (13 ml to 15 ml) and nasopharyngeal spray (2 ml) twice a day. There were 98 patients in a randomized control group who did not receive the treatment of chlorhexidine.
The charts were further reviewed to determine the patients' status upon discharge. Deaths caused by COVID-19 infection were identified and mortality was calculated. There was no outpatient follow-up available regarding subsequent fatalities following discharge due to COVID-19 infection.
Study-2 methods
Patients from november 2021 through January 2022: In our study focused on the efficacy of chlorhexidine in eradicating oropharyngeal SARS-CoV-2, there were instances where certain patient data were not included in the initial publication. These specific patient records pertained to cases that emerged subsequent to the conclusion of the primary study. To address this, a retrospective review was conducted on the unpublished patient data, which has been designated as "Study 2."
The study for those patients with COVID-19 infection were from November 2021 through January 2022 when the Delta and Omicron variants were dominant. The method of study was the same as the previous one for patients in 2020 and 2021, during which the dominant circulating viruses were the initial one from Wuhan, China and the Delta variant from India [1]. We checked whether chlorhexidine could also significantly reduce the mortality rate for COVID-19 infection during this period.
Disclosures: The study was conducted according to the guidelines of the declaration of Helsinki and approved by the ethics committees of the Pipeline Health (El Segundo, CA) and AHMC Healthcare (Alhambra, CA) hospital systems. All participating patients were given an explanation of the study and signed consent forms.
Patients: Patients were referred to us for infectious diseases consultation from Los Angeles Metropolitan Hospitals.
Patients had a laboratory-confirmed diagnosis of COVID-19, based upon a positive COVID-19 test by Nucleic Acid Amplification Test (NAAT), positive rapid antigen test and/or PCR test [2] with clinical evidence of pulmonary infiltration and hypoxia to justify hospitalization.
The testing materials for both the antigen test and NAAT were supplied by Abbott Laboratories. Certified hospital technologists administered these tests. The PCR test was performed using the real-time Reverse Transcriptase-Polymerase Chain Reaction (rRT-PCR) method, employing the cobas 6800 SARS-CoV-2 test from Roche Molecular Systems in Branchburg, NJ. The PCR testing was conducted at WestPac Labs in Los Angeles, CA. Most of our patients (67 out of 70 patients) received oxygen therapy and steroids. Only 9 patients received remdesivir. All patients received anticoagulants and antibiotics [3-5].
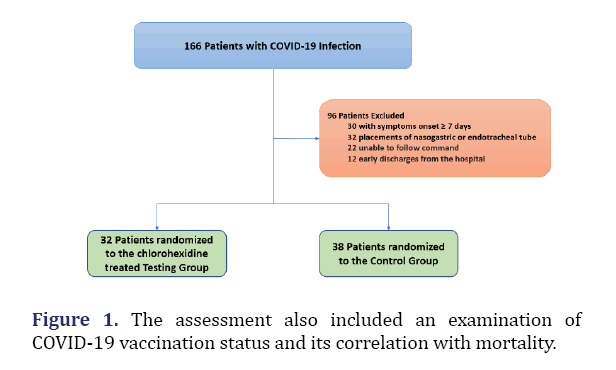
The age range of patients was from 23 to 85 years old. Demographically, the majority of patients were of either Hispanic or Asian ethnic background. There was a total of 70 patients enrolled in this study. An alternative randomized control was used for this study, with 32 patients in the chlorhexidine treatment group and 38 patients in the control group. The study excluded patients demonstrating a mentally altered state or patients who were on either respiratory intubation or nasopharyngeal tubes, or BIPPA. As previously detailed, 13-15 ml of 0.12% chlorhexidine was given by oral rinse and 2 ml of 0.12% chlorhexidine was given through nasopharyngeal spray [1].
The efficacy of chlorhexidine in eradicating SARS-CoV-2 from the oropharynx was evaluated. The efficacy of chlorhexidine in reducing the mortality of COVID-19 infection was also calculated. Persistence of oropharyngeal SARS-CoV-2 and mortality of COVID-19 infections. We also evaluated the effect of a persistent viral presence in the oropharynx on mortality. This study compared the difference in mortality between viral eradication or persistence in the oropharynx, with or without chlorhexidine application.
Statistical methodology
All relevant statistical analysis was performed in Microsoft Excel (version 2103). In examining the data gathered from each of our two independent studies, we performed a two-proportion z-test to analyse the data provided by both the test and control groups to fit a standard normal distribution. In doing so, we utilized a benchmark p-value of p ≤ 0.05 to determine the statistical significance of our findings. All data was rounded to two significant figures.
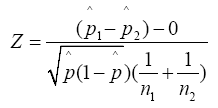
Quality control of the study
Our study complied with the equator network guideline (22).
Results
Study-1 results
Chlorhexidine treatment influence on COVID-19 mortality from 2020 through the early period of 2021: The data indicates that 117 patients were treated with chlorhexidine and 22 of those patients died due to COVID-19 infections, resulting in a mortality rate of 18.8%. In the control group, 98 patients were not treated with chlorhexidine and 40 of those patients died due to COVID-19 infections, resulting in a mortality rate of 40.82%. Therefore, the efficacy of chlorhexidine in reducing COVID-19 deaths for inpatients was 53.93%. Overall, patient mortality was significantly lower when they were given chlorhexidine treatments compared to when they were not. The difference between the chlorhexidine treatment group and the control group was statistically significant (p ≤ 0.05) (Table 1).
| Patients | Total number | Number of death | Mortality rate |
|---|---|---|---|
| Chlorhexidine treated | 117 | 22 | 18.80% |
| Control | 98 | 40 | 39.70% |
Mortality in chlorhexidine treated group is 22/117 (18.8%). Mortality in control group is 40/98 (39.7%). Efficiency of chlorhexidine in reducing COVID-19 mortality is (1-18.8/39.7)=52.65%.
Statistical analysis: The mortality difference between the chlorhexidine treatment group and control group was significantly different (p ≤ 0.05).
Study-2 results
Chlorhexidine influence on COVID-19 mortality from November 2021 through January 2022.
Eradication of SARS-CoV-2 from oropharynx: We attempted to evaluate the efficacy of chlorhexidine in eradicating SARS-CoV-2 from the oropharynx. The chlorhexidine treatment group had 32 patients and the control group, which did not receive chlorhexidine treatment, had 38 patients. In the treatment group, 22 patients, representing 68.75% of the sample, tested negative for COVID-19 after four days of treatment with chlorhexidine. In the control group, 8 patients, representing 21% of the sample, tested negative for COVID-19 after four days of hospitalization. As such, the percentage of patients testing negative for COVID-19 after four days was significantly higher in the group that received chlorhexidine treatments compared to the group that did not. The difference in the results between the chlorhexidine treatment group and the control group was statistically significant (p ≤ 0.05).
Chlorhexidine effect on the mortality of COVID-19 infection (November 2021 through January 2022): The data indicates that 32 patients were treated with chlorhexidine and 7 of those patients died due to COVID-19 infections, resulting in a mortality rate of 21.9%. In the control group, 38 patients were not treated with chlorhexidine and 17 of those patients died due to COVID-19 infections, resulting in a mortality rate of 44.7%. Therefore, the efficacy of chlorhexidine in reducing mortality of inpatient COVID-19 infected patients was 51%. Overall, patient mortality was significantly lower when they were given chlorhexidine treatments compared to when they were not (p ≤ 0.05).
A further point to note is that the correlation between COVID-19 vaccination status and mortality was also evaluated. Among the 32 patients in the chlorhexidine treatment group, 2 of the 12 vaccinated individuals died (Table 2). Furthermore, among the 38 patients in the control group, 5 of the 15 vaccinated individuals died (Figure 1).
| Patients | Total number | Number of death | Mortality rate |
|---|---|---|---|
| Chlorhexidine treated | 32 | 7 | 21.90% |
| Control | 38 | 17 | 44.70% |
Mortality in Chlorhexidine treated group is 7/32(21.9%). Mortality in control group is 17/38(44.7%). Efficiency of chlorhexidine in reducing COVID-19 mortality is (1-21.9/44.7)=52%.
Statistical analysis: The mortality difference between the chlorhexidine treated and control group was significantly different (p ≤ 0.05) (Table 3).
| Groups | Vaccinated patients | Number of deaths | Mortality rate |
| Treatment Group | 12 | 2 | 16.67% |
| Control Group | 15 | 5 | 33.33% |
The effect of mortality by SARS-CoV-2 persistence in the oropharynx group without chlorhexidine treatment: There were 151 patients in the control group who did not receive treatment with 0.12% chlorhexidine. Of this group, 25 patients tested negative for COVID-19 four days after admission. Four deaths were reported, resulting in a mortality rate of 16%. In contrast, 126 patients tested positive for COVID-19 four days after admission and 53 deaths were reported, resulting in a 42% mortality rate. Therefore, there is a reduction of mortality if virus disappeared from the oropharynx: (1-16/42)=61.9%. The difference in mortality rates between the two groups is statistically significant (p ≤ 0.05).
Group with chlorhexidine treatment: There were 161 patients in the treatment group who received four days of treatment with 0.12% chlorhexidine. Of this group, 125 patients tested negative for COVID-19 four days after-treatment. There were 20 deaths, resulting in a mortality rate of 16.0%. In contrast, 36 patients tested positive for COVID-19 four days after treatment and there were 8 deaths, resulting in a 22.2% mortality rate. Therefore, there is a reduction of mortality if virus disappeared from the oropharynx: (1-16/22.2)=28.8%. However, the difference in mortality rates between the two groups is not statistically significant. The effect of chlorhexidine on SARS-CoV-2 persistence in the oropharynx and associated mortality were given in (Tables 4 and 5).
| Control group | Number of survivors | Number of deaths | Mortality rate |
|---|---|---|---|
| Virus persisted | 126 | 53 | 42.06% |
| Virus subsided | 25 | 4 | 16% |
| Treatment group | Number of survivors | Number of deaths | Mortality rate |
|---|---|---|---|
| Virus persisted | 36 | 8 | 22.22% |
| Virus subsided | 125 | 20 | 16% |
Note: Reduction of mortality if virus disappeared from the oropharynx: (1-16/22.22)=28.8% The difference in mortality rate is not statistically significant.
Discussion
We previously demonstrated that chlorhexidine can be used as a powerful agent in eradicating SARS-CoV-2 from the oropharynx, which, if administered to patients in a timely manner, could help prevent COVID-19 infections [1]. In our current study, we repeated this study to further confirm the effectiveness of chlorhexidine in eradicating SARS-CoV-2 from the oropharynx.
Our current study further suggests that chlorhexidine is able to reduce COVID-19 inpatient mortality by about half. The vaccination status does not appear to interfere with our study, as the vaccinated and unvaccinated groups were of equal size.
In our current study, we also demonstrated that the disappearance of SARS-CoV-2 in the oropharynx significantly reduces COVID-19 mortality. However, with the added chlorhexidine treatment, there was no statistically significant difference in mortality rates between the absence or persistent presence of the virus in the oropharynx. This further supports the benefit of chlorhexidine as an agent in reducing the mortality of COVID-19 infection. However, if the number of patients in the study were to increase, we may see a statistical significance in mortality even with the addition of chlorhexidine.
As a result, continued monitoring of SARS-CoV-2 test results in the oropharynx can be used as an indicator to predict the prognosis of the disease. A recent study indicated that higher upper respiratory and serum SARS-CoV-2 viral load or antigen levels had a higher mortality from COVID-19 infection [6,7]. This might explain why persistent oropharyngeal SARS-CoV-2 results in higher mortalityin our study and highlights the importance of eradicating SARS-CoV-2 from the oropharynx. If chlorhexidine treatments fail to completely eliminate SARS-CoV-2 in the oropharynx, we still recommend continuing chlorhexidine treatments since mortality in the chlorhexidine-treated group tended to be lower than the group without the treatment of chlorhexidine.
We performed this study during different viral outbreak periods, including the original Wuhan SARS-CoV-2 viral outbreak of 2020 through early 2021 and the Delta and early Omicron viral outbreaks in late 2021 through early 2022. The results were similar, indicating that chlorhexidine is also effective even if there is a viral mutation.
Our study was limited to the initial four days of hospitalization, which covers the highest viral load period of SARS-CoV-2 during hospitalizations [8,9]. The potential further benefit of chlorhexidine beyond the initial four days of hospitalization is unknown at present. However, we recommend continuing chlorhexidine treatment during the entire hospital course, as chlorhexidine has no significant side effects.
The application of chlorhexidine in our study was limited to oral rinse and oropharyngeal spray. For maximal efficacy, nasal instillation of the drug should also be considered [10].
In our experience, the administration of a chlorhexidine oropharyngeal spray in addition to an oral rinse could lead to improved results. If an oropharyngeal spray is not available, we suggest a thorough chlorhexidine rinse of the oropharyngeal area by gargling. We also recommend that providers instruct patients not to ingest chlorhexidine into their stomach.
The recently developed oral antimicrobial agents, nirmatrelvir/ritonavir and molnupiravir, have been used to prevent mild or moderately severe COVID-19 infections from progressing to severe infections. However, these antimicrobial agents are used for outpatient treatment and should only be used within the first 5 days of initial sickness. Additionally, nirmatrelvir/ritonavir and molnupiravir have potential side effects and drug-drug interactions. The possibility of SARS-CoV-2 developing resistance to these drugs is also a concern [11-14]. Nirmatrelvir/Ritonavir and molnupiravir are effective in the early stage of the disease, specifically during an upper respiratory infection. Similarly, chlorhexidine has been found to eradicate the virus in the upper respiratory tract, which may partially explain its effectiveness in reducing COVID-19 mortality.
Another agent used in treating COVID-19 infections is remdesivir. However, it should be noted that this drug is mainly effective in the early stages of the disease.
Chlorhexidine has been used to prevent aspiration pneumonia [15,16] and as a treatment for dental infections [17]. Peri-implant mucositis [18], as well as a pre-operative agent to prevent surgical infections [19]. Accounting for the high mortality in our control group despite following the generally recommended treatment for COVID-19 infection might be due to more severe cases of COVID-19 infection in our hospitals. Moreover, during the most intense stages of the pandemic in Los Angeles, there was a mass shortage of available beds in metropolitan hospitals across the county. As a result, the hospitals where we based our experiments transformed their parking lots into temporary COVID-19 wards. Patients referred to us for infectious disease consultation were usually sicker patients. Milder cases were not referred to us. We also had criteria for selecting patients for the study, such as patients with NG tube, or not able to follow command to take chlorhexidine which might have influenced the mortality rate of the study patients. Other confounding factors in mortality include the patient's racial background [20,21] and insurance status. Since our study was a randomized control study, this results of ourstudy.
Conclusion
Our previous study indicated that chlorhexidine can eradicate SARS-CoV-2 from the oropharynx, which potentially prevents the spread of disease to others. The current study shows that the use of 0.12% chlorhexidine via oral rinse and oropharyngeal spray can also reduce the mortality rate of COVID-19 infected patients by half. Furthermore, we also found that the persistence of SARS-CoV-2 in the oropharynx beyond four days is an ominous sign for disease prognosis. Finally, we conclude that chlorhexidine is a useful prophylactic and therapeutic agent for the treatment of outpatients and inpatients with COVID-19.
Limitation
The study is a randomized control study instead of a double blind control study. We have not completed control clinical study to prove chlorhexidine in preventing SARS-CoV-2 infected patients from spreading to other people. We did not have enough patients to do the study on vaccinated patients.
Conflict of Interest Statement
The authors declare that there are no conflict of interests.
References
- Huang YH, Huang JT. Use of chlorhexidine to eradicate oropharyngeal SARS‐CoV‐2 in COVID‐19 patients. J Med Virol 2021;93(7):4370-4373.
[Crossref] [Google Scholar] [PubMed]
- Use of chlorhexidine to eradicate oropharyngeal SARS‐CoV‐2 in COVID‐19 patients.
- Crossref
- Cantini F, Niccoli L, Nannini C, Matarrese D, Di Natale ME, Lotti P, et al. Beneficial impact of baricitinib in COVID-19 moderate pneumonia; multicentre study. J of Infec 2020;81(4):647-679.
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19. N Engl J Med 2020;383(19):1813-1826.
[Crossref] [Google Scholar] [PubMed]
- Fajnzylber J, Regan J, Coxen K, Corry H, Wong C, Rosenthal A, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat commun 2020;11(1):5493.
[Crossref] [Google Scholar] [PubMed]
- Rogers AJ, Wentworth D, Phillips A, Shaw-Saliba K, Dewar RL. The association of baseline plasma SARS-CoV-2 nucleocapsid antigen level and outcomes in patients hospitalized with COVID-19. Ann Intern Med 2022;175(10):1401-1410.
[Crossref] [Google Scholar] [PubMed]
- Puhach O, Meyer B, Eckerle I. SARS-CoV-2 viral load and shedding kinetics. Nat Rev Microbiol 2023;21(3):147-161.
- Hakki S, Zhou J, Jonnerby J, Singanayagam A, Barnett JL, Madon KJ, et al. Onset and window of SARS-CoV-2 infectiousness and temporal correlation with symptom onset: A prospective, longitudinal, community cohort study. Lancet Respir Med 2022;10(11):1061-1073.
[Crossref] [Google Scholar] [PubMed]
- Basu S, Holbrook LT, Kudlaty K, Fasanmade O, Wu J, Burke A, et al. Numerical evaluation of spray position for improved nasal drug delivery. Sci Rep 2020;10(1):10568.
[Crossref] [Google Scholar] [PubMed]
- Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med 2022;386(15):1397-1408.
[Crossref] [Google Scholar] [PubMed]
- Fact sheet for health care providers: Emergency use authorization for molnupiravir. FDA 2021.
- Abraham S, Nohria A, Neilan TG, Asnani A, Saji AM, Shah J, et al. Cardiovascular drug interactions with nirmatrelvir/ritonavir in patients with COVID-19: JACC review topic of the week. J Am Coll Cardiol 2022;80(20):1912-1924
[Crossref] [Google Scholar] [PubMed]
- Twenter P. Pfizer's antiviral drug could result in 'paxlovid mouth'. Becker's Hospital Review 2023.
- Munro CL, Grap MJ, Jones DJ, McClish DK, Sessler CN. Chlorhexidine, toothbrushing and preventing ventilator-associated pneumonia in critically ill adults. Am J Crit Care 2009;18(5):428-437.
[Crossref] [Google Scholar] [PubMed]
- Houston S, Hougland P, Anderson JJ, LaRocco M, Kennedy V, Gentry LO. Effectiveness of 0.12% chlorhexidine gluconate oral rinse in reducing prevalence of nosocomial pneumonia in patients undergoing heart surgery. Am J Crit Care 2002;11(6):567-570.
[Crossref] [Google Scholar] [PubMed]
- Brookes ZL, Bescos R, Belfield LA, Ali K, Roberts A. Current uses of chlorhexidine for management of oral disease: A narrative review. J Dent 2020;103:103497.
[Crossref][Google Scholar] [PubMed]
- Butera A, Pascadopoli M, Pellegrini M, Gallo S, Zampetti P, Cuggia G, et al. Domiciliary use of chlorhexidine vs. postbiotic gels in patients with peri-implant mucositis: A split-mouth randomized clinical trial. Applied Sciences 2022;12(6):2800.
- Anderson DJ, Podgorny K, Berríos-Torres SI, Bratzler DW, Dellinger EP, Greene L, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 35(S2):S66-S88.
[Crossref] [Google Scholar] [PubMed]
- Gold JA, Rossen LM, Ahmad FB, Sutton P, Li Z, Salvatore PP, et al. Race, ethnicity and age trends in persons who died from COVID-19-United States, May-August 2020. Morb Mortal Wkly Rep 2020;69(42):1517.
[Crossref] [Google Scholar] [PubMed]
- Enhancing the quality and transparency of health research. Equator Network 2023.